Proteoglycan Signaling in Inflammation, Fibrosis, and Cancer


Liliana Schaefer (Principal Investigator), Madalina V. Nastase, Jinyang Zeng-Brouwers, Chiara Poluzzi, Tzung-Harn Louise Hsieh, Heiko Rödig, Riad Haceni
Liliana Schaefer is a Deputy Director of the Institute of Pharmacology and Toxicology at the Goethe University in Frankfurt/Main, President of the International Society for Matrix Biology (www.ismb.org), Councilor of the Histochemical Society (http://histochemicalsociety.org/), Vice Chair & Chair-elect of the Research Gordon Conference on Proteoglycans (https://www.grc.org/conferences.aspx?id=0000233), Deputy Editor of Matrix Biology (http://www.journals.elsevier.com/matrix-biology) journal, Co-editor of Cellular Signalling (https://www.journals.elsevier.com/cellular-signalling/editorial-board), and Associate Editor of the Journal of Histochemistry & Cytochemistry (http://jhc.sagepub.com).
Research program:
Soluble matrix components (= danger signals signifying tissue injury) as novel therapeutic targets in inflammation, fibrosis, and cancer
In recent years it has become increasingly clear that extracellular matrices (ECMs) are far more than merely structural scaffolds that help to organize cells into tissues by providing mechanical support. Instead, ECMs are highly dynamic macromolecular assemblies that exert profound influence on cell behavior. This is due to both, receptor-dependent interactions of cells with resident components of the ECM (such as collagens, laminins, and fibronectin) as well as to cell signaling by transiently bound mediators (growth factors, cytokines, and chemokines). Our laboratory has investigated the role of the two small leucine-rich proteoglycans (SLRPs), decorin and biglycan, in inflammation, innate immunity and renal fibrosis. We have made significant contributions to the field of innate immunity by discovering that both SLRPs, when present in their soluble forms in blood and bodily fluids, can act as endogenous “danger” signals and thus become directly involved in modulating the activity of Toll-like receptors (TLRs) and the NLRP3 inflammasome. Soluble biglycan and decorin, two endogenous ligands of TLR2 and TLR4, induce the production of TNF-α, IL-12 and pro-IL-1β cytokines via NF-κB activation in macrophages. By interacting with the P2X7/P2X4 receptor, biglycan induces the assembly of the NLRP3 inflammasome and activation of Caspase-1, leading to maturation and secretion of IL-1β. Decorin inhibits the binding of TGF-β1 to its receptor, thereby suppressing the maturation of miR-21, a posttranscriptional inhibitor of PDCD4. Consequently, enhanced PDCD4 levels block the translation of the anti-inflammatory cytokine IL-10 (Fig. 1).
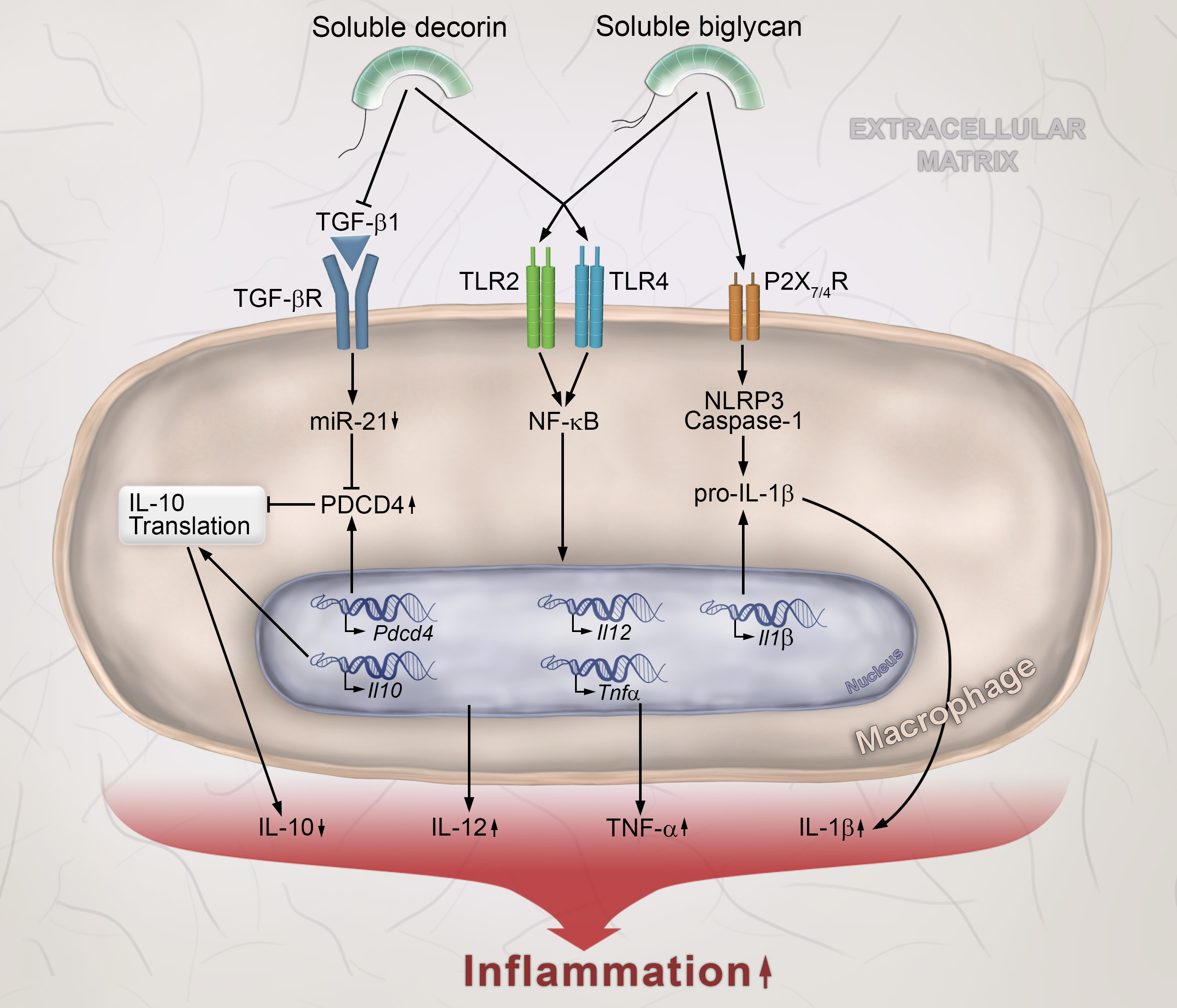
Figure 1: Soluble SLRPs autonomously trigger or potentiate inflammatory signaling in macrophages. Bryant.., Schaefer et al., Crit Rev Biochem Mol Biol 10: 1-21, 2015
Our group is particularly interested in the contribution of the ECM in renal inflammatory disorders. In Germany alone, 12.000 new cases of end-stage renal failure are reported every year. The management of chronic kidney disease (CKD) represents a huge burden to healthcare systems as terminal kidney failure develops, requiring renal replacement therapies such as dialysis and kidney transplantation. CKD frequently leads to renal fibrosis. This process involves excessive accumulation of ECM components, leading to glomerulosclerosis, tubulointerstitial fibrosis, and finally to irreversible loss of renal function and end-stage renal disease. Major factors involved in the evolution of fibrosis are Angiotensin II (Ang II), Transforming Growth Factor-β1 (TGF-β1), and Connective Tissue Growth Factor (CTGF) (Fig. 2).
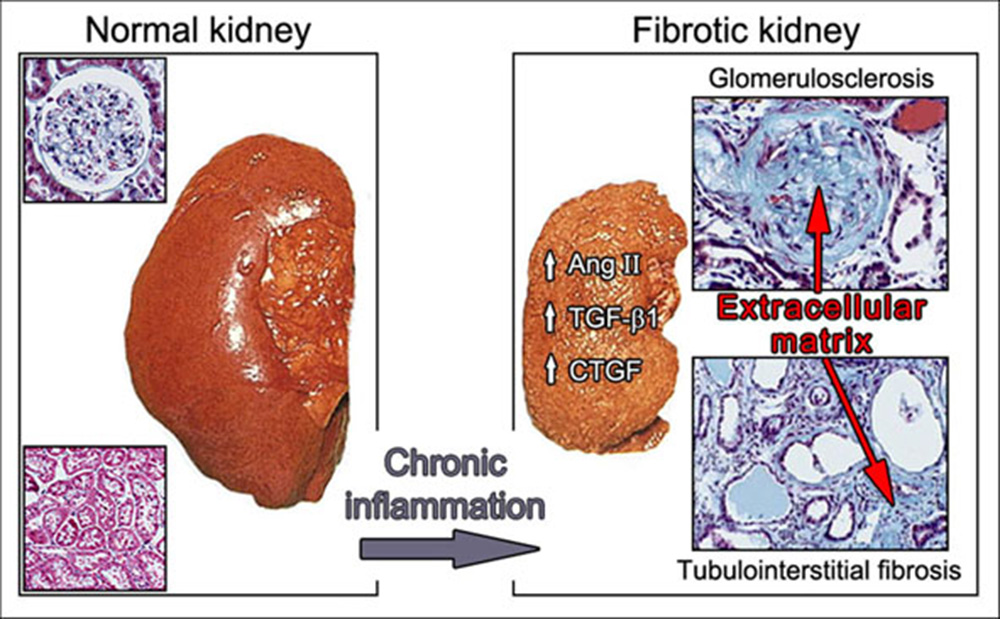
Figure 2: Chronification of renal inflammation.
We demonstrated that in renal disorders, SLRPs are essential elements that regulate several pathophysiological processes including fibrosis, inflammation and tumor progression. Decorin has remarkable antifibrotic and antitumorigenic properties and is considered a valuable potential treatment of these diseases. Biglycan crucially influences renal inflammation and is a potential target in the treatment of inflammatory conditions. Biglycan is synthesized at mRNA level in renal diseases by macrophages and renal resident cells in response to IL-1β, IL-6, TNF-α and TGF-β, PDGF, respectively. This newly synthesized biglycan binds to the ECM. As the underlying disease advances, the ECM at some point will become saturated and unable to further sequester the excess of biglycan. Consequently, synthesized biglycan will then be released into the extracellular space in its soluble form. Simultaneously, proteinases may cleave ECM-bound biglycan and thereby release even more soluble biglycan, which in turn will interact with both macrophages and renal resident cells. In macrophages, biglycan binds to TLR2 and TLR4 and induces the production of TNF-α and pro-IL-1β cytokines. By interacting with P2X7 biglycan induces the assembly of the NLRP3 inflammasome and activation of caspase-1, which in turn leads to maturation of IL-1β. Biglycan also induces the production of chemokines and recruitment of the respective immune cells via either the TLR2/TLR4/MyD88 or TLR4/TRIF pathways. In renal resident cells, biglycan induces the synthesis of the Fbn1 gene and thus production of fibrillin-1.
In addition, soluble biglycan and its fragments might spill over into the blood stream and thus reach other organs or might even be brought back to the kidney via the circulation.
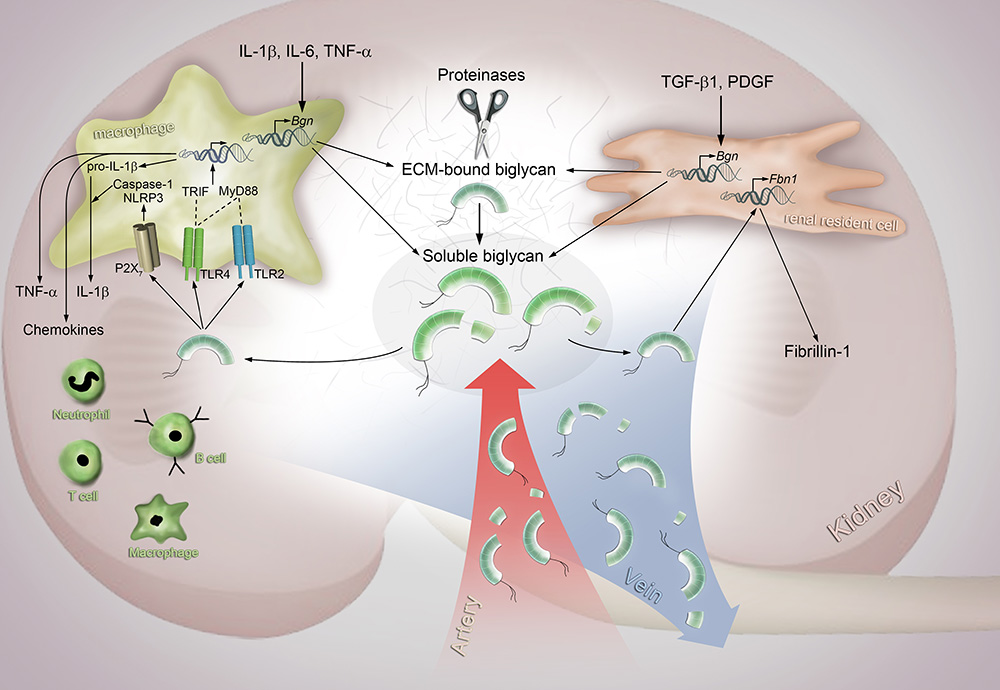
Figure 3: Fate of biglycan in renal injury. Hsieh et al., Int J Biochem Cell Biol 54: 223-235, 2014
Bgn, biglycan; Fbn1, fibrillin-1; IL-6, interleukin 6; IL-1β, interleukin 1β; MyD88, myeloid differentiation primary response 88; NLRP3, NLR family, pyrin domain–containing 3; PDGF, platelet-derived growth factor; TGF-β, transforming growth factor-β; TLR, toll-like receptor; TNF-α, tumor necrosis factor beta; TRIF, TIR-domain-containing adaptor-inducing interferon beta.
Our research is supported by:





Selected Original Publications:
1. Nastase MV, Zeng-Brouwers J, Beckmann J, Tredup C, Christen U, Radeke HH, Wygrecka M, Schaefer L: Biglycan, a novel trigger of Th1 and Th17 cell recruitment into the kidney. Matrix Biol doi: 10.1016/j.matbio.2017.12.002
2. Nastase MV, Zeng-Brouwers J, Frey H, Hsieh LT, Poluzzi C, Beckmann J, Schroeder N, Pfeilschifter J, Lopez-Mosqueda J, Mersmann J, Ikeda F, Iozzo RV, Dikic I, Schaefer L: An essential role for SHARPIN in the regulation of caspase 1 activity in sepsis. Am J Pathol 186: 1206-1220, 2016
Press release: Novel Research Lays the Groundwork for New Therapies Against Sepsis highlights the protective role for SHARPIN discovered by Nastase et al.
3. Frey H, Moreth K, Hsieh LTH, Zeng-Brouwers J, Rathkolb B, Fuchs H, Gailus-Durner V, Iozzo RV, Hrabě de Angelis M, Schaefer L: A novel biological function of soluble biglycan: Induction of erythropoietin production and polycythemia. Glycoconj J DOI: 10.1007/s10719-016-9722-y, 2016
4. Hsieh LT, Frey H, Nastase MV, Tredup C, Hoffmann A, Poluzzi C, Zeng-Brouwers J, Manon-Jensen T, Schröder K, Brandes RP, Iozzo RV, Schaefer L: Bimodal role of NADPH Oxidases in the regulation of biglycan-triggered IL-1β synthesis. Matrix Biol 49: 61-81, 2016
5. Zeng-Brouwers J, Beckmann J, Nastase MV, Iozzo RV, Schaefer L: De novo expression of circulating biglycan evokes an innate inflammatory tissue response via MyD88/TRIF pathways. Matrix Biol 35: 132-142, 2014
6. Moreth K, Frey H, Hubo M, Zeng-Brouwers J, Nastase MV, Hsieh L T-H, Haceni R, Pfeilschifter J, Iozzo RV, Schaefer L: Biglycan-triggered TLR-2- and TLR-4-signaling exacerbates the pathophysiology of ischemic acute kidney injury. Matrix Biol 35: 143–151, 2014
7. Merline R, Moreth K, Beckmann J, Nastase MV, Zeng-Brouwers J, Tralhão JG, Lemarchand P, Pfeilschifter J, Schaefer RM, Iozzo RV, Schaefer L: Signaling by the matrix proteoglycan decorin controls inflammation and cancer through PDCD4 and miR-21. Sci Signal. 4, ra75, 2011
8. Ikeda F, Deribe YL, Skånland SS, Stieglitz B, Grabbe C, Franz-Wachtel M, van Wijk S, Goswami P, Nagy V, Terzic J, Tokunaga F, Androulidaki A, Nakagawa T, Pasparakis M, Iwai K, Sundberg JP, Schaefer L, Rittinger K, Macek B, Dikic I: SHARPIN forms a linear ubiquitin ligase complex regulating NF-κB activity and apoptosis. Nature 471: 637-641, 2011
9. Moreth K, Brodbeck R, Babelova A, Gretz N, Spieker T, Zeng-Brouwers J, Pfeilschifter J, Young M, Schaefer RM, Schaefer L: The proteoglycan biglycan regulates expression of the B cell chemoattractant CXCL13 and aggravates murine lupus nephritis. J Clin Invest 120: 4251-4272, 2010
10. Babelova A, Moreth K, Tsalastra-Greul W, Zeng-Brouwers J, Eickelberg O, Young MF, Bruckner P, Pfeilschifter J, Schaefer RM, Gröne H-J, Schaefer L: Biglycan: a danger signal that activates the NLRP3 inflammasome via a Toll-like- and P2X-multireceptor complex. J Biol Chem 284: 24035-24048, 2009
11. Schaefer L, Babelova A, Kiss E, Hausser HJ, Baliova M, Krzyzankova M, Marsche G, Young MF, Mihalik D, Gotte M, Malle E, Schaefer RM, Grone HJ: The matrix component biglycan is proinflammatory and signals through Toll-like receptors 4 and 2 in macrophages. J Clin Invest 115: 2223-2233, 2005
More publications of Liliana Schaefer can be found at Google Scholar
Recent Reviews:
1. Nastase MV, Janicova A, Roedig H, Hsieh LTH, Wygrecka M, Schaefer L: Small leucine-rich proteoglycans in renal inflammation: two sides of the coin. J Histochem Cytochem 66:261-272, 2018
2. Frevert CW, Felgenhauer J, Wygrecka M, Nastase MV, Schaefer L: Danger associated molecular patterns from the extracellular matrix provide temporal control of innate immunity. J Histochem Cytochem 66: 213–227, 2018
3. Nastase MV, Zeng-Brouwers J, Wygrecka M, Schaefer L: Targeting renal fibrosis: mechanisms and drug delivery systems. Adv Drug Deliv Rev doi.org/10.1016/j.addr.2017.12.019
4. Poluzzi C, Iozzo RV, Schaefer L: Endostatin and endorepellin: a common route of action for similar angiostatic cancer avengers. Adv Drug Deliv Rev 97: 156-173, 2016
5. Iozzo RV, Schaefer L: Proteoglycan form and function: A comprehensive nomenclature of proteoglycans. Matrix Biol 42: 11-55, 2015
6. Schaefer L. Complexity of danger: The diverse nature of damage-associated molecular patterns. J Biol Chem 289: 35237-35245, 2014
CONTACT
Prof. Dr. med. Liliana Schaefer
Allgemeine Pharmakologie und Toxikologie
Division Nephropharmakologie
Klinikum der Goethe Universität
Theodor-Stern Kai 7, Bau 74 – 3.108a
60590 Frankfurt a.M.
Germany
Tel: (+49) 069-6301-7899
Fax: (+49) 069-6301-83027
Email: Schaefer@med.uni-frankfurt.de